Immunology and Inflammation
Inflammation is a common element underlying most diseases, including cancer. The inflammatory condition is associated with an activated immune system, including activated immune cells and biomolecules. Inflammatory models of diseases are therefore critical to the understanding of disease etiology across colitis, psoriasis, multiple sclerosis, arthritis, and fibrosis.
At Aragen, we employ animal models that show tissue-specific inflammation and utilize a variety of approaches to evaluate novel treatments. Our preclinical research in immunology and inflammation focuses on arthritis and autoimmunity. Aragen scientists have expertise in preclinical investigations of the joints and lung functions in vivo, including bronchoalveolar lavage, cytology, immunohistochemistry, and analysis of biochemical, immunological, and molecular parameters. Strong collaborations with various academic, government and industrial laboratories have enabled the development of several experimental models of immunology and inflammatory diseases.
- Inflammatory Fibrosis
- Bleomycin-induced idiopathic pulmonary fibrosis (IPF) in mouse and rats
- Carbon tetrachloride (CCl4)-induced liver fibrosis
- Dermal Inflammation
- Imiquimod-induced psoriasis in mice
- IL-23-induced psoriasis in mice
- Oxazolone-induced ear/skin inflammation in mice
- Delayed-type hypersensitivity in mice
- Multiple Sclerosis
- MOG 35-55-induced experimental autoimmune encephalitis (EAE) in mice
- Arthritis
- Collagen-induced arthritis: DBA/1 mice, Lewis rats
- Adjuvant-induced arthritis: rats
- Lung Disease
- Asthma
- Acute lung injury models
- Inflammatory Bowel Disease
- Dextran sodium sulphate (DSS)-induced colitis model
- Citrobacter-induced colitis in mice
- Repeated Autoimmunity/Inflammation
- EAE model: SJL mouse, Lewis rats
- Lupus: NZBxNZW F1 mouse, MRL/LPR
- Other Inflammation/Allergy Models
- Anaphylaxis model in mice
- Endotoxemia models in mice and rats
- Peritonitis in mice and rats
- AOM/DSS model of colitis-associated cancer
In Vitro Pharmacology Services
Aragen’s in vitro pharmacology services facilitate lead identification and optimization and are part of our integrated drug discovery solutions from target validation through preclinical candidate selection. To support these services, we offer a broad range of biochemical and cellular assays and have deep experience in many therapeutic areas, including oncology, immuno-oncology, inflammation, autoimmunity, fibrosis, cardiovascular and metabolic diseases. A key part of our in vitro services is the identification, validation, and screening of biomarkers. Our In vitro Pharmacology services utilize isolated cells, tissues, and organs to assess the pharmacological properties of drugs.
Assay Development & High-Throughput Screening
Biochemical and cell-based assays employing recombinant proteins, normal and engineered cell lines as well as reporter cell systems can be performed for a wide range of biological target classes. Reagents for assay development and screening may be either commercially sourced or custom-made in our labs. Multimode, multiplex, phenotypic, label-free, and radiometric microplate-based screening platforms provide support for a wide range of high-throughput screening campaigns.
Assay Platforms
- Luminescence
- Time-resolved fluorescence resonance energy transfer (TR-FRET)/ homogeneous time resolved fluorescence (HTRF®)
- Fluorescence Polarization
- Absorbance
- AlphaScreen®
- High Content Imaging
- Flow Cytometry
- NanoBRET™
- qPCR
- Surface Plasmon Resonance (SPR-BiaCore)
- Automated Western Blot (Bio-Techne/Protein Simple)
- Radiometric (SPA, filter binding)
- Light Scattering
- Patch Clamp
- Next Generation Sequencing
Target Classes
- Enzymes, GPCRs
- Ion Channels
- Transcription Factors
- Kinases
- Transporters
- Nuclear Hormone Receptors
Assay Capability Highlights
- Receptor binding assays: Measuring selectivity and affinity between potential therapeutics and specific receptors
- Cell-based assays: Assessing drug effects on various cellular processes or signaling pathways using cultured cells (obtained from commercial sources or client-supplied)
- In vitro toxicology assays (Toxvit™): Multiparametric cytotoxicity solutions
- Immunophenotyping: Characterization and quantification of different immune cell populations in response to therapeutic intervention
- Antibody response: Therapeutic intervention that may involve the stimulation or modulation of antibody responses.
- Inflammation and tissue damage assays: Assess inflammation modulators to reduce tissue damage
- Immunomodulatory effects on immune responses and immune activity
- Pharmacokinetics and pharmacodynamics (PK/PD)
- Cellular activation and proliferation assays
- Immune cell function assays
- Enzyme activity assays
- Cytokine profiling
- Safety Evaluation
Non GLP Assays
Toxvit™: Multi-Parametric Cytotoxicity
- IC50 determination
- Cell loss/death (fluorescent imaging)
- Nuclear size and morphology (fluorescent imaging)
- ~50 different cell lines of human origin available for cytotoxicity testing
Immuno-oncology Specific Assays at Aragen
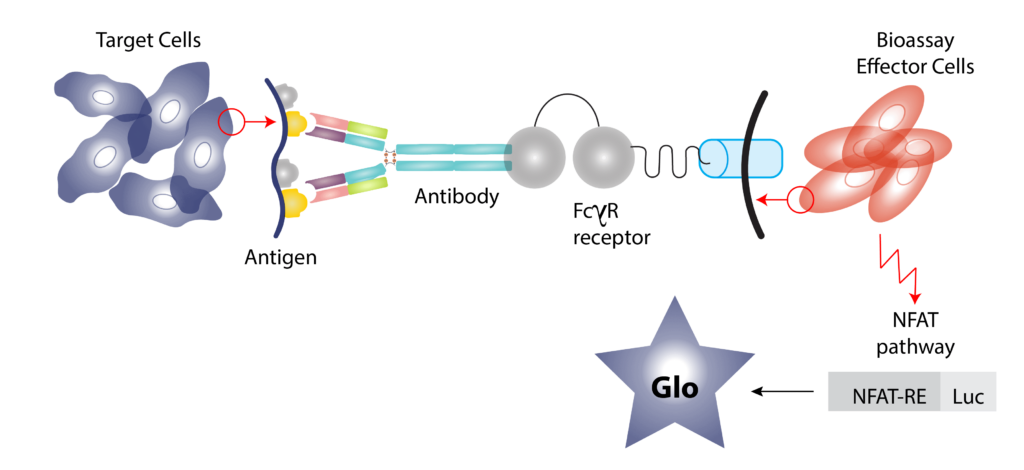
- Mechanisms of study for antibody drugs include Antigen Dependent Cellular Cytotoxicity (ADCC), Antigen Dependent Cellular Phagocytes (ADCP) and Complement Dependent Cytotoxicity (CDC).
- We employ both primary cells and target expressing cell lines for ADCC/ADCP assessment by a reporter bioassay.
- CDC assessed using normal human serum as the source of complement.
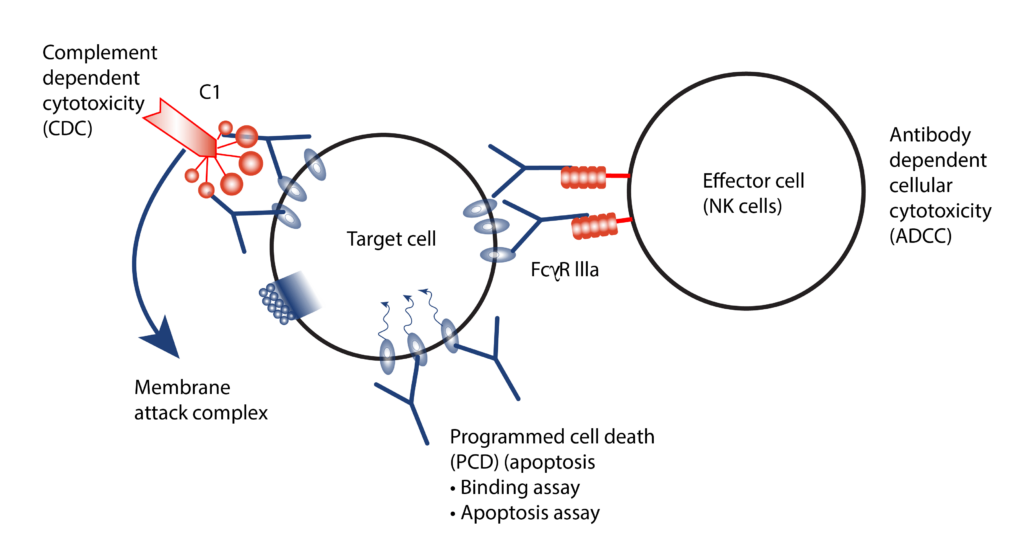
In vitro Immunological Assays
Summary
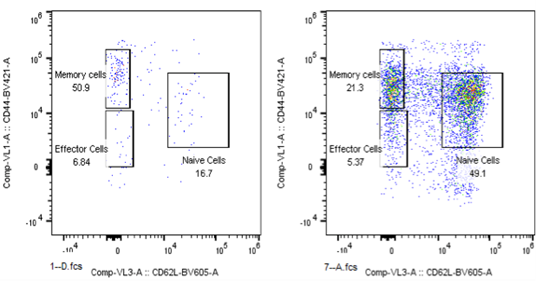
Analysis of Effector and Memory Markers in CD8+ T cell subsets in blood sample from CT26 Bearing Mouse (Left) or Tumor Free Survivor after the treatment (Right).
- Analyzed both human and mouse immunophenotyping markers by FACS
- Human PBMC engraftment (CD45+ CD3+)
- Antigen binding assay
- Determination of T cell activation/proliferation (CFSE)
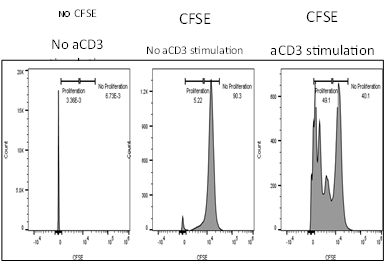
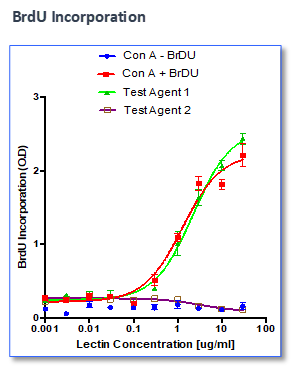
Summary
Effect of test agents or anti-CD3/anti-CD28 antibodies on PBMC
- PBMCs were plated in presence/absence of test agents or anti-CD3/anti-CD28 antibodies
- Proliferation is measured by BrdU incorporation or CFSE incorporation
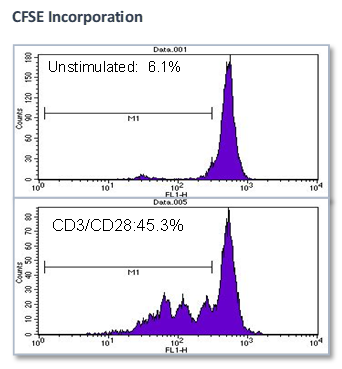
Summary
Analysis of antibody responses measured in cells.
- PBMCs mixed with CFSE labeled target cells at a fixed E:T ratio
- Serial dilutions of test antibodies were added to cells
- Flow cytometry to measure percentage of remaining viable target cells
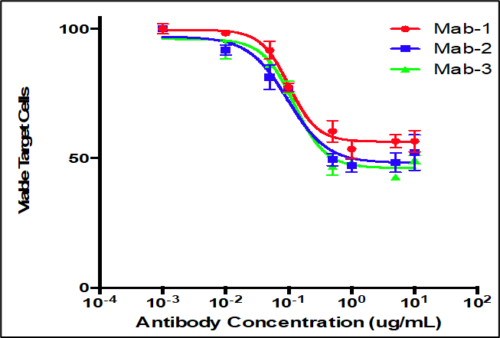
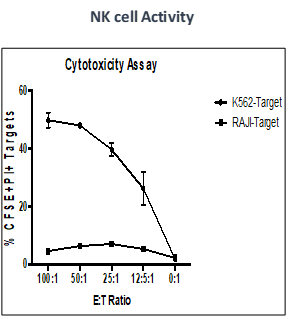
Summary
Effect of test peptide on human myelogenous leukemia cells (K562) and human Burkitt’s lymphoma cells (RAJI).
- Measured by LDH release from target cells (K562 and RAJI).
- Effector CTLs are generated from PBMCs (multiple donors) using specific peptides
- Target cells killed by PBMCs -measured by calculating percentage of CFSE+/PI+
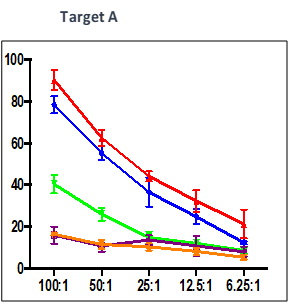
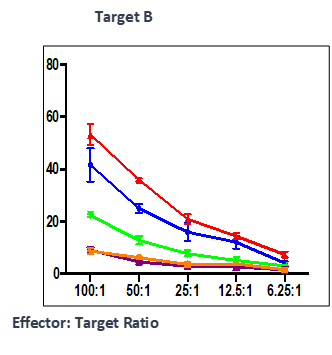
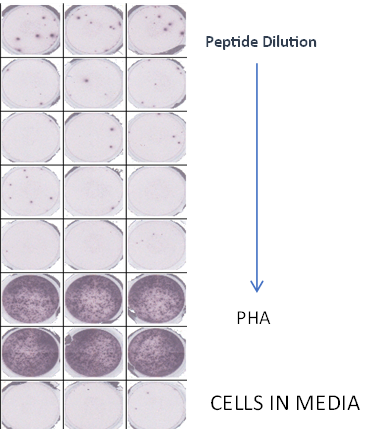
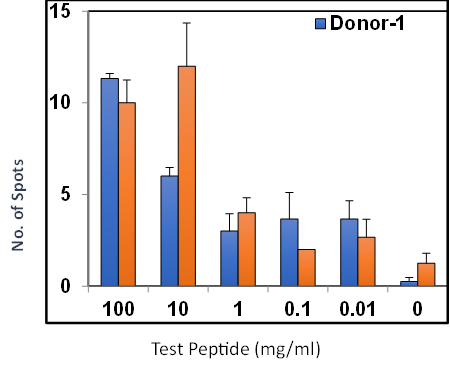
Summary
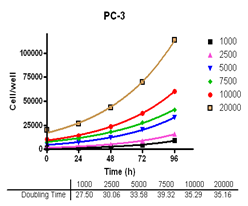
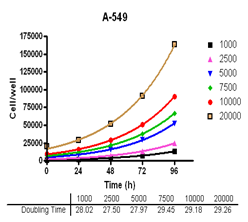
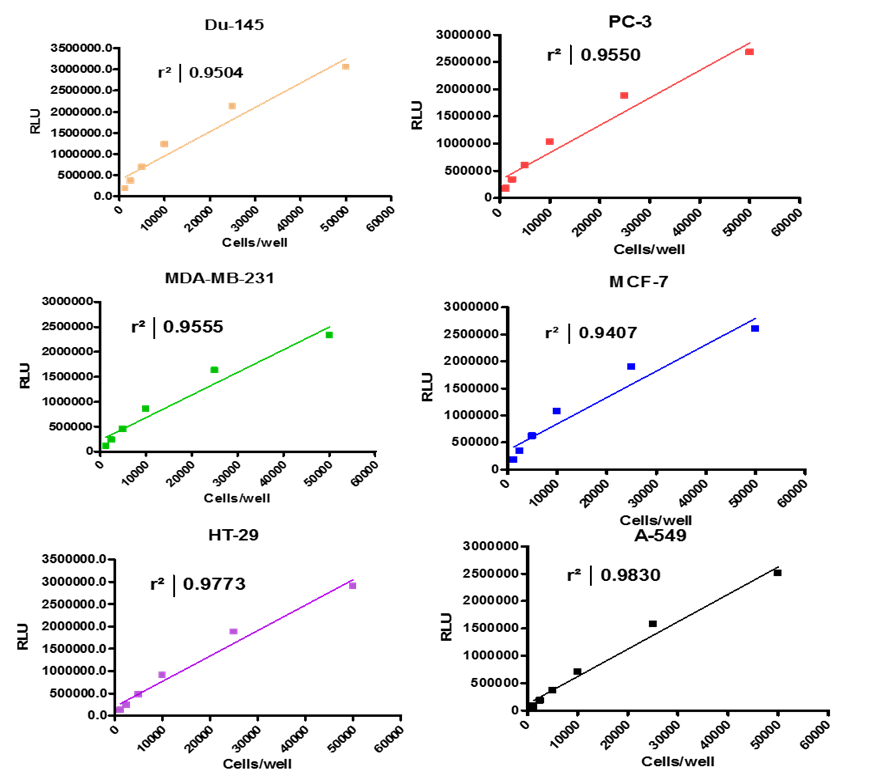
Analyze viable cell number and Cell Titre-Glo reagent sensitivity in Du-145, PC-3, MDA-MB-231 and MCF-7, HT-29 and A-549 cells.
Summary
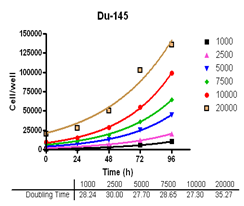
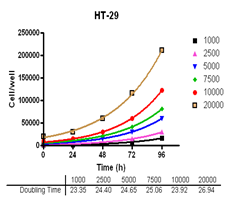
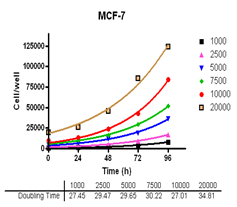
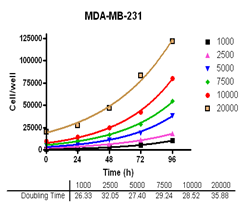
Evaluate cell growth and doubling time in Du-145, PC-3, MDA-MB-231 and MCF-7, HT-29 and A-549 cells.