Upstream Process Flow
A
B
C
D
E
F
Our goal is to provide bioproduction flexibility, providing well-validated process parameters and ensuring consistency and scalability of client’s biotherapeutics. We use nonproprietary commercially available media and feed for screening studies, allowing transfer to any CDMO (Contract Development and Manufacturing Organization) as desired. We scale carefully, screening media and feed in shake flasks, followed by process parameter screening and optimization in 1L bioreactors and then process consistency and scalability studies in 5L bioreactors.
Aragen’s High Throughput Lab Accelerates Upstream Process Development
Aragen utilizes Sartorius Ambr® 250 automated bioreactor systems for high-throughput process development and late-stage clone evaluation. These are integrated with Nova Biomedical Flex2 cell culture analyzers and Beckman Coulter Vi-CELL cell viability analyzers. This fully automated system enables Aragen scientists to perform parallel fermentation and cell culture development and accelerating the development process. Sophisticated Design of Experiment (DOE) models provide the means to quickly screen process parameters like pH, DO, Temp and agitation. Cell culture performance with those parameters as well as CO2 metabolites, VCD and percent viability, are tracked throughout the process.

Sartorius Ambr® 250 automated bioreactor
Case Studies: Process Development to Increase Titer & Improve Product Quality
Aragen scientists improved titer from 20 to 300ug/mL with >85% fully formed protein.
Reduced Western Blot from daily samples from Bioreactor Run
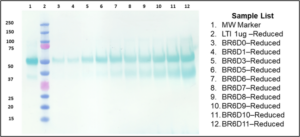
- Media adaption
- Shake flask fed batch evaluation with different media and feed.
- Scalable and consistent data from shake flask to 1L and 5L Bioreactor in terms of Viable Cell Density (VCD) and titer profile
- Glycan profile, SEC (Size Exclusion Chromatography), cIEF, Mass Spec, SPR (Surface Plasmon Resonance) is comparable to Originator Anti-inflammatory Tumor Necrosis Factor Inhibitor
Part A: Media Adaptation; Growth Profile
- Master Cell Bank (MCB) received from a client was thawed, scaled up in the original medium, Media A, based on Aragen protocol.
- SP2/0 cells were adapted in different mediums. The SP2/0 adapted well (viability >90%) with Media B, D and F. These media were used in the next stage.
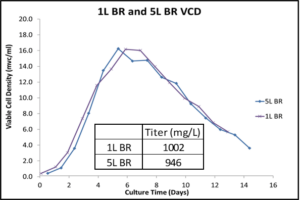
The following graphs shows the Shake Flask Quality (SFQ) Optimization for VCD, Titer in 1L and 5L bioreactors (BR)
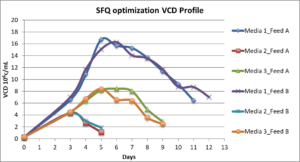
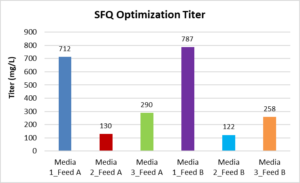
Comparison Titre and VCD between 1L and 5L BRs over 16 days of cell culture
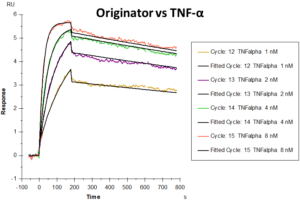
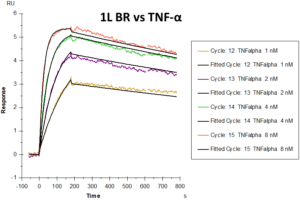
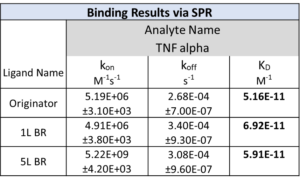
Graphs showing differential responses to different feed-cycles- Originator versus TNF-Alpha in 1L BR, and Table showing binding parameters of TNF-Alpha in 1L and 5L BR.
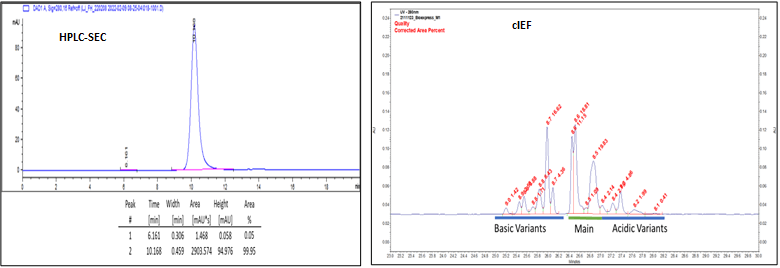
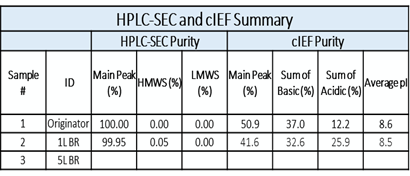
HPLC-SEC and cIEF analyses of Protein Samples: cIEF analysis showing the basic variant peaks along with Main and Acidic Variants’ peaks. Summary of Protein analysis shown in the Table below.
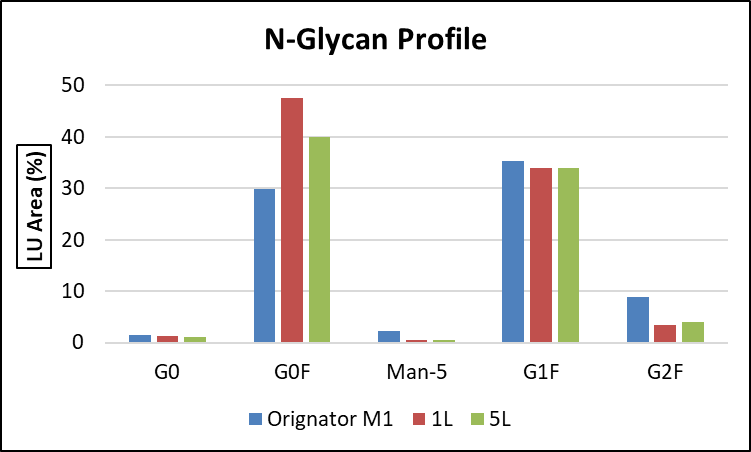
Comparison of N-Glycan Profile between the Originator and the biosimilar processed in 1L and 5L BRs.
Downstream Process Development (DSP)
Downstream processing is a series of operations that includes primary recovery and capture, viral inactivation, concentration, and formulation. Downstream process development can be challenging, with multiple steps requiring optimization to ensure both robustness of the process and quality of the product. Aragen’s DSP experts will navigate the development challenges for your product, whether it is a monoclonal antibody, or a complex biologic that may have a greater range of process- and product-related impurities that will need to be removed.
After the initial harvest and filtration, the first chromatographic capture step isolates the product from the bulk of impurities, such as cell culture media components and proteases. This step helps to concentrate and stabilize the product. One or more polishing chromatography steps will remove remaining impurities of the target product. At each step, a process risk assessment is performed to identify how operating parameters may affect the product’s critical quality attributes.
Typical key steps of DSP
- Harvest and Filtration-Depth filtration, Hollow Fibers
- Primary Capture- Affinity and Ion exchange
- Viral inactivation- Low pH
- Buffer Exchange and concentration
- Formulation
Wash
- Additives
- pH, conductivity
- Wash Volume (CV)
- Flow rate, temp
- Monitoring of pH
- Conductivity, UV
Elution
- Pressure, pH
- Conductivity, Conc
- Volume, flow rate
- Temp, buffers and salt
- Resin Stability
- Gradient profiles
Strip
- Additives, pH
- Conductivity
- Wash volume
- Flow rate
- Temp, buffers and salt
- Resin Stability
Wash
- pH, conductivity
- Wash volume
- Flow rate
- Temp, buffers and salt
- Resin Stability
- Additives
Purity
- Yield
- Host Cell Protein
- Host Cell DNA
Risk Ranking for Polishing Steps
Column Factors
- Bed Height of the Column
- Resin
- Flow Rate
- Temperature
Equilibration
- pH
- Conductivity
- Volume
Load
- Conc, pH
- Conductivity
- Temperature
- Protein Load
- Hold time
Chase
- PH
- Conductivity
- Peak cut
Parameters
- Yield
- HCP
- Purity
Contact Us to Learn More
Contact our team in the Morgan Hill, California facility to learn more about Aragen’s process development services and how we can take your project from concept all the way to having an IND-enabling data package.
